Use of CBCT Imaging, Open-Source Modeling Software, and Desktop Stereolithography 3D Printing to Duplicate a Removable Dental Prosthesis—A Proof of Concept
Wei-Shao Lin, DDS; Bryan T. Harris, DMD; and Dean Morton, BDS, MS
Abstract
This article describes an alternative computer-aided design/computer-aided manufacturing (CAD/CAM) technique that uses cone-beam computed tomography (CBCT) imaging, open-source 3-dimensional (3D) modeling software, and a desktop stereolithography (SLA) 3D printer to duplicate an existing complete removable dental prosthesis (CRDP). Intended to offer a proof of concept, this proposed technique provides dental clinicians who have access to a CBCT imaging unit and a desktop SLA 3D printer an option to manufacture a duplicated prosthesis in-office. The 3D model of the CRDP can be preserved indefinitely, and the CAD/CAM process makes it possible to manufacture the CRDP multiple times with ease, when necessary. Different materials can be selected for the SLA 3D printer, and the duplicated prosthesis could be used for a variety of clinical and laboratory indications, such as a custom tray, a trial prosthesis, a reference record for a definitive prosthesis in laboratory procedures, or a radiographic template. Clinicians should select and use 3D software, printers, and materials with proper biocompatibility and classification and clearance from respective medical device regulatory bodies (such as US Food & Drug Administration) in different intended applications.
Additive manufacturing (AM), often referred to as 3-dimensional (3D) printing, has been considered a disruptive technology and brought significant socio-economic, environmental, geopolitical, security, and intellectual property implications to industry.1,2 Based on the definition from the American Society for Testing and Materials, AM is a process of “joining materials to make objects from 3D model data, usually layer upon layer, as opposed to subtractive manufacturing methodologies.”3 The distinguishing feature of the AM process from both subtractive manufacturing (ie, cutting, milling, and grinding)4 and traditional formative manufacturing (ie, pressing, casting, and forming) is that it enables a layer-wise production and is suitable for manufacturing devices or products with individualized, increased geometric complexity.5
AM encompasses an array of manufacturing technologies, including stereolithography (SLA), selective laser sintering inkjet printing, fused deposition modeling (FDM), selective electron beam melting, etc.6-9 First pioneered by Charles W. Hull in 1986, SLA produces 3D models by constructing successive 2-dimensional (2D) layers of light-polymerizing liquid photopolymer upon each other, with each layer being polymerized by a concentrated beam of ultraviolet light.6,7
First adopted by the automobile and aerospace industries for prototyping, AM has now been used extensively in medicine and dentistry. In dentistry, AM can be used to fabricate dental casts, preproduction wax or polymer patterns for dental prostheses, molds for dental or facial prostheses, and metal or all-ceramic prostheses or their framework.10,11 When used in conjunction with 3D imaging modalities, such as cone-beam computed tomography (CBCT), computed tomography (CT), or magnetic resonance imaging (MRI), and 3D virtual modeling and planning software, AM can create patient-specific craniofacial casts, surgical templates, intraoperative guidance devices, and/or reconstruction plates.12-14
To generate 3D objects with the AM process from a 3D imaging volumetric dataset in the data imaging and communications in medicine (DICOM) file format, two types of software systems are often required.15-18 A 3D modeling software can be used to convert DICOM files into a universally accepted 3D file format, such as standard tessellation language (STL) or wavefront object (OBJ),17,18 and/or to further modify or edit the 3D file.1,5 A 3D slicing software can then alter the orientation of the file in the 3D printer and divide it into thin slice datasets suitable for the subsequent AM process.18 With the increasing accessibility of open-source 3D software and the decreasing cost of desktop 3D printers, clinicians now have opportunities to incorporate 3D design and printing in local dental clinics without having to outsource these tasks to external production manufacturers.19
An acceptable complete removable dental prosthesis (CRDP) or diagnostic tooth arrangement can be duplicated for a variety of clinical indications, such as a custom tray or trial prosthesis or a reference record to facilitate obtaining a definitive impression, maxillomandibular relationship, occlusal vertical dimension, and diagnostic tooth arrangement for a conventional or implant-supported dental prosthesis.20-23 In addition, the duplicated CRDP/diagnostic tooth arrangement can be used in conjunction with radiopaque markers as a radiographic template to indicate the desired position and angulation of the implant during radiographic imaging acquisition. The radiographic template can then be modified and used as a surgical template to allow for a prosthetically driven osteotomy and/or the placement of dental implants.24-26 Different material and technique combinations have been proposed to duplicate the CRDP or the diagnostic tooth arrangement with autopolymerizing acrylic resin, such as creating the mold with irreversible hydrocolloid impression material and impression trays, or using a reline jig and polyvinyl siloxane with dental stone, or polyvinyl siloxane only.27-29 A recent report proposed the use of a handheld optical scanner (Artec Space Spider, Artec 3D, artec3d.com), a custom-made rotary table, proprietary 3D software (Artec Studio 9.0, Artec 3D), and an FDM 3D printer (MakerBot Replicator 5th Generation, MakerBot, makerbot.com) to duplicate the existing CRDP.30
This article describes a proof of concept using an in-office CAD/CAM workflow of CBCT imaging, open-source modeling software, and a desktop SLA 3D printer to duplicate an existing CRDP.
Technique
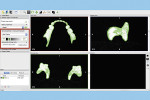
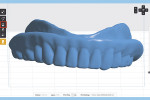
The process of duplicating an existing CRDP with this technique begins with placing the CRDP on an appliance holder (3D Accuitomo 170, J. Morita USA, global.morita.com) and polystyrene block, and scanning the CRDP with the CBCT imaging scanner (3D Accuitomo 170) at fields of view of 80 mm x 80 mm, 75 kV, and 2.0 mA (Figure 1). The scanned volumetric dataset is then saved in the DICOM file format.
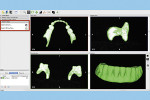
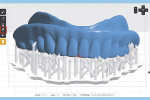
The next step is to download and install the open-source 3D modeling software systems (InVesalius 3.0, CTI Renato Archer, cti.gov.br; and Autodesk Meshmixer 3.2, Autodesk, meshmixer.com).1,5,17,18 After initiating the InVesalius 3.0 software, select “Load data” in the left panel window to import the DICOM volumetric dataset. Then choose “Select region of interest” and select “Soft Tissue” from the dropdown menu of “Set predefined or manual threshold” and click the “Create surface” icon (Figure 2). Next, choose “Export data,” and select “Export 3D surface” from the menu and save the 3D model in an STL format (Figure 3).
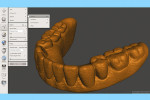
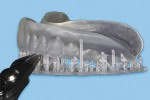
At this point, initiate the Meshmixer 3.2 software and, in the left panel window, choose “Select” function to select the entire 3D model. Select the “Deform” and “Smooth” options to create a 3D model with a smoother surface finish (Figure 4). Choose the “Shape Preserving” option under the “Smoothing Type” function and then choose a Smoothing Value of 1, a Smoothing Scale of 4, and Constraint Rings of 3 (Figure 5). Select “Export” from the menu and save the 3D model in an STL format.
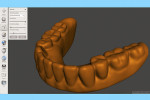
Next, import the STL file into the 3D slicing software (PreForm Software, Formlabs, Inc., formlabs.com) of the desktop SLA 3D printer (Form 2, Formlabs, Inc.). In the software, on the left panel menu, select the “Orientation” tab and orient the 3D model with the intaglio surface facing up (Figure 6). Then, select the “Supports” tab on the left panel menu to automatically generate the supporting structures for the subsequent 3D manufacturing process (Figure 7). The complete 3D model file then gets transferred to the desktop SLA 3D printer, and appropriate light-polymerizing liquid photopolymer material (Standard Clear Resin, Formlabs, Inc.) can be used to additively manufacture the 3D object. For intraoral usage, biocompatible light-polymerizing liquid photopolymer material (such as, Dental SG Resin, Formlabs, Inc.) should be selected and used.
After removing the manufactured object and build plate from the printer (Figure 8), take the object from the build plate and place it in a plastic container filled with 91% isopropyl alcohol to rinse off residual light-polymerizing liquid photopolymer (Figure 9). Place the manufactured object in a dental light-polymerizing unit, such as the Enterra™ VLC Curing Unit (Dentsply International, dentsplysirona.com), for 20 minutes (or as per the light-polymerizing material manufacturer’s recommendations) to ensure complete post-manufacture polymerization (Figure 10).
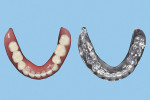
Then, use cutting pliers to separate the supporting structures from the duplicated CRDP (Figure 11), and finish and polish the unit with laboratory instruments (Ultra Denture Kit, Brasseler USA, brasselerusadental.com), as necessary (Figure 12 and Figure 13).
With an appropriate selection of liquid photopolymer material, the duplicated CRDP could then be used for different indications, such as a custom tray for a definitive prosthesis, a trial prosthesis, a reference record for a definitive prosthesis in laboratory procedures, or a radiographic template.
Discussion
This article describes a proof of concept for dental clinicians to duplicate a CRDP with in-office digital data acquisition (CBCT imaging) and a CAD/CAM (SLA) process. The article can also serve as a reference for clinicians who have access to a CBCT imaging unit and desktop SLA 3D printer to digitalize a desired object and additively manufacture it in-office, without outsourcing to a dental laboratory.
In a previous study,4 the CAD/CAM fabrication process was shown to produce the most accurate and reproducible CRDP base adaptation when compared with more traditional pack-and-press, pour, and injection processing techniques. When compared with the corresponding preprocessing cast, the maxillary CAD/CAM CRDP bases had a range of mean deviation from -6.1 µm (palatal region, standard deviation [SD] = 25.1 µm) to 21.5 µm (apex of denture border region, SD = 33.1 µm). Using the same surface matching software (Geomagic Control X Software, 3D Systems, 3dsystems.com) in this present report as in the previous study, measurements were made at the intaglio and occlusal surfaces of the duplicated CRDP, using the original CRDP as the reference. The mean deviations and SDs of the duplicated CRDP were 0.087 µm ± 0.941 µm (range, 6.308 µm to -18.056 um) for the intaglio surface, and -0.237 µm ± 2.126 um (range, 14.590 µm to -15.1996 µm) for the occlusal surface (Figure 14 and Figure 15). Although further studies will be needed to prove the validity of the proposed technique, the finding from this report may provide initial information to promote future clinical application and research interest of in-office digital data acquisition using the CBCT imaging method and the CAD/CAM process using open-source 3D software and a desktop 3D printer.
Traditional techniques to duplicate an acceptable CRDP or diagnostic tooth arrangement typically involve multiple manual steps, including creating the mold for a duplicated prosthesis with various impression materials, injecting materials into the mold, and recontouring and polishing the duplicated prosthesis.27-29 These multiple procedures may affect the accuracy and consistency of the duplicated prosthesis. Depending on the selected technique or material to create the mold for the duplication process, the mold is either indicated only for single-use or short-term storage (eg, if irreversible hydrocolloid impression material is used) or may consume more material and be costly to fabricate (eg, if a polyvinyl siloxane impression or special laboratory equipment is used).28 If the initial duplicated prosthesis is not adequate or multiple duplicated prostheses are needed, it may be time-consuming and labor-intensive to repeat the duplication process. The 3D model of a desired CRDP, however, can be preserved indefinitely and the CAD/CAM process makes it possible to easily manufacture the desired object multiple times.
In this technique, a free open-source 3D modeling software was used to create a 3D model of the desired object from the DICOM dataset. Although various 3D modeling software systems may be used to convert DICOM volumetric data to universally accepted 3D file formats in STL or OBJ, high initial costs and continuous update fees may be incurred with some proprietary software.18,30 Different free open-source software systems that are intuitive even to individuals with no engineering background have gained popularity in the medical community.17,18 The open-source 3D modeling software systems demonstrated in this article allow the user to eliminate undesired areas in the DICOM volumetric dataset by adjusting the images with pre-set thresholds17,18 and create smoother surface finishing of the desired 3D object1,5 while prompting the user to follow on-screen instructions with an easy-to-use interface.
A desktop SLA 3D printer was used in this proposed technique to manufacture a CAD/CAM-duplicated prosthesis in-office. When clinicians have access to a desktop 3D printer, the opportunity is there to additively manufacture objects in the office with a high degree of convenience and independence from commercial dental laboratories or production centers. One previous report described the use of a desktop FDM 3D printer to duplicate an existing CRDP.30 The FDM process is similar to the working concept of a glue gun. The string of molten material is extruded from the nozzle and forms the layers of the desired object as the material cools and hardens. The FDM process is mostly used as an economical at-home 3D printer with lower finish quality and surface resolution.6-8 When compared to FDM, the SLA 3D printer (Form 2) used in this report can produce objects with higher strength and a smoother surface finish, albeit at a higher material cost.8,18 The manufactured object will require sterilization for clinical applications in medicine and dentistry. Common sterilization techniques include high-temperature, such as steam; chemical, such as ethylene oxide or hydrogen peroxide; and radiation.9 Most objects additively manufactured with polymers will require non-heat sterilization to avoid distortion. The desktop SLA 3D printer used in this technique also affords utilization of a wide variety of available light-polymerizing photopolymers, including a newly developed biocompatible, autoclavable polymer (Dental SG Resin), and it can be used for radiographic template indications at a higher material cost. Dental clinicians can select appropriate light-polymerizing photopolymers and an appropriate sterilization method based on the desired application of the manufactured object.
The primary limitation of this proposed technique is the accessibility and affordability of the CBCT imaging unit and desktop SLA 3D printer. The technique is more suitable for a facility where the required equipment is readily available, such as larger group dental practices or academic institutions. If clinicians do not have access to a desktop SLA 3D printer, an alternative approach is to transfer the obtained 3D model file to a commercial dental laboratory or centralized CAD/CAM facility for the production. It is also possible to obtain the digital data acquisition of the desired object with a dental laboratory scanner. However, for most clinicians, a CBCT imaging unit may be more accessible than a dental laboratory scanner. Additionally, although the use of pre-set thresholds has been shown to be an efficient, user-friendly way to reliably and accurately recreate 3D anatomic models in the free open-source 3D modeling software used in this report, future clinical studies will be needed to validate its indication for creating 3D prosthesis models in conjunction with a desktop SLA 3D printer.17
Lastly, but importantly, dental technicians and clinicians who are interested in in-house 3D printing should have adequate knowledge of CAD/CAM systems and medical device regulatory bodies, and, when necessary, utilize 3D software, printers, and materials with proper biocompatibility and respective medical device regulatory bodies’ (such as FDA in the United States) classification and clearance in different intended applications.
Summary
The duplicated prosthesis was created using the technique described, ie, an in-office, digital method using CBCT imaging, free open-source 3D modeling software programs, and a desktop SLA 3D printer. This technique may provide clinicians who have access to a CBCT imaging unit and a desktop SLA 3D printer better use of available technology and equipment for in-house manufacturing. However, it is of paramount importance that dental technicians and clinicians using the in-house 3D printing technique select and utilize design and manufacturing processes/materials that conform with recommendations from manufacturers and guidelines from respective medical device regulatory bodies for various intended applications.
About the Authors
Wei-Shao Lin, DDS
Associate Professor
Division of Prosthodontics
Department of Oral Health and Rehabilitation
University of Louisville School of Dentistry
Louisville, Kentucky
Bryan T. Harris, DMD
Associate Professor
Division of Prosthodontics
Department of Oral Health and Rehabilitation
University of Louisville School of Dentistry
Louisville, Kentucky
Dean Morton, BDS, MS
Chair and Professor
Department of Prosthodontics
Indiana University School of Dentistry
Indianapolis, Indiana
References
1. Berman B. 3-D printing: The new industrial revolution. Bus Horiz. 2012;55(2):155-162.
2. Campbell TA, Ivanova OS. Additive manufacturing as a disruptive technology: implications of three-dimensional printing. Technol Innov. 2013;15(1):67-79.
3. Guo N, Leu MC. Additive manufacturing: technology, applications and research needs. Front Mech Eng. 2013;8(3):215-243.
4. Goodacre BJ, Goodacre CJ, Baba NZ, Kattadiyil MT. Comparison of denture base adaptation between CAD-CAM and conventional fabrication techniques. J Prosthet Dent. 2016;116(2):249-256.
5. Gao W, Zhang Y, Ramanujan D, et al. The status, challenges, and future of additive manufacturing in engineering. Computer-Aided Des. 2015;69:65-89.
6. Shafiee A, Atala A. Printing technologies for medical applications. Trends Mol Med. 2016;22(3):254-265.
7. van Noort R. The future of dental devices is digital. Dent Mater. 2012;28(1):3-12.
8. Wong KV, Hernandez A. A review of additive manufacturing. ISRN Mech Eng. 2012;2012:1-11.
9. Mitsouras D, Liacouras P, Imanzadeh A, et al. Medical 3D printing for the radiologist. Radiographics. 2015;35(7):1965-1988.
10. Sun J, Zhang FQ. The application of rapid prototyping in prosthodontics. J Prosthodont. 2012;21(8):641-644.
11. Torabi K, Farjood E, Hamedani S. Rapid prototyping technologies and their applications in prosthodontics, a review of literature. J Dent (Shiraz). 2015;16(1):1-9.
12. Farré-Guasch E, Wolff J, Helder MN, et al. Application of additive manufacturing in oral and maxillofacial surgery. J Oral Maxillofac Surg. 2015;73(12):2408-2418.
13. Peng Q, Tang Z, Liu O, Peng Z. Rapid prototyping-assisted maxillofacial reconstruction. Ann Med. 2015;47(3):186-208.
14. Scherer MD. Presurgical implant-site assessment and restoratively driven digital planning. Dent Clin North Am. 2014;58(3):561-595.
15. Marro A, Bandukwala T, Mak W. Three-dimensional printing and medical imaging: a review of the methods and applications. Curr Probl Diagn Radiol. 2016;45(1):2-9.
16. Hieu LC, Zlatov N, Vander Sloten J, et al. Medical rapid prototyping applications and methods. Assemb Autom. 2005;25(4):284-292.
17. Poleti ML, Fernandes TM, Pagin O, et al. Analysis of linear measurements on 3D surface models using CBCT data segmentation obtained by automatic standard pre-set thresholds in two segmentation software programs: an in vitro study. Clin Oral Investig. 2016;20(1):179-185.
18. Chae MP, Rozen WM, McMenamin PG, et al. Emerging applications of bedside 3D printing in plastic surgery. Front Surg. 2015;2:25.
19. Fullerton JN, Frodsham GC, Day RM. 3D printing for the many, not the few. Nat Biotechnol. 2014;32(11):1086-1087.
20. Soo S, Cheng AC. Complete denture copy technique—A practical application. Singapore Dent J. 2014;35:65-70.
21. Lin WS, Ozdemir E, Morton D. A three-appointment alternative treatment protocol for fabricating an implant-supported milled bar overdenture. J Prosthet Dent. 2012;107(2):75-79.
22. Ozkomur A, Manfroi F. Multifunctional guide for implant placement, impressions, and an occlusal index for fixed complete dentures. J Prosthodont. 2016 Mar 9. doi: 10.1111/jopr.12472.
23. Inokoshi M, Kanazawa M, Minakuchi S. Evaluation of a complete denture trial method applying rapid prototyping. Dent Mater J. 2012;31(1):40-46.
24. Bidra AS. Technique for systematic bone reduction for fixed implant-supported prosthesis in the edentulous maxilla. J Prosthet Dent. 2015;113(6):520-523.
25. Zitzmann NU, Marinello CP. Treatment plan for restoring the edentulous maxilla with implant-supported restorations: removable overdenture versus fixed partial denture design. J Prosthet Dent. 1999;82(2):188-196.
26. Arfai NK, Kiat-Amnuay S. Radiographic and surgical guide for placement of multiple implants. J Prosthet Dent. 2007;97(5):310-312.
27. Habib SR, Vohra FA. Replacing existing dentures by copy-denture technique for geriatric patients: a case report. JPDA. 2013;22(4):265-270.
28. Plummer KD, Nahon M. Use of a reline jig to fabricate a complete denture implant surgical guide from an existing complete denture. J Prosthet Dent. 2004;92(6):598-599.
29. Sukotjo C, Radics A. Use of vinyl polysiloxane for the fabrication of implant surgical guide. J Prosthet Dent. 2004;92(6):596-597.
30. Kurahashi K, Matsuda T, Goto T, et al. Duplication of complete dentures using general-purpose handheld optical scanner and 3-dimensional printer: Introduction and clinical considerations. J Prosthodont Res. 2017;61(1):81-86.